Colloidal gold is a colloidal gold that is polymerized into gold particles of a specific size under the action of a reducing agent and forms a stable colloidal state due to electrostatic action. The common preparation methods of colloidal gold solution are roughly classified into white phosphorus reduction method, sodium borohydride reduction method, ascorbate reduction method, trisodium citrate reduction method and trisodium citrate reduction method. The basic principle is to add an appropriate amount of reducing agent to a certain concentration of gold solution to reduce gold ions to gold atoms. The gold particles of different diameters required can be obtained by changing the ratio of chloroauric acid to reducing agent in the reaction system.
1. Colloidal gold mark
Under alkaline conditions, the surface of the colloidal gold particles is negatively charged and can form a strong bond with the electrostatic attraction of a non-covalent bond between the positively charged groups of the target protein. This binding has no biological activity on the labeled protein. Significant impact. The antigen or antibody adsorbed on the surface of the colloidal gold can be directed to carry the colloidal gold particles to the location of the corresponding antibody or antigen on the tissue or cells, on the solid support. Due to the high electron density of the gold particles, when these markers aggregated at the antigen-antibody reaction to a certain density (ie, gold particles of 107/mm2), visible pink spots appeared.
The colloidal gold labeling requires optimization of the pH environment, protein/antibody concentration, and parameters. The method used is the concentration gradient method.
A optimum pH selection
pH is the key determinant of the labeling process. Generally, the labeling effect is best in the environment where the pI of the protein/antibody is slightly alkaline. The pH of the colloidal gold solution can be adjusted by adding 0.1 M potassium carbonate or 0.1 N hydrochloric acid. At the same time, because the colloidal gold solution may damage the probe, precision pH test paper is often used for pH determination. During the operation, adjust the colloidal gold solution to 3~10 pH gradients, add the labeled protein/antibody, mix and let stand for 15 min at room temperature, then add 10% sodium chloride at room temperature for 15 min, and record the lowest pH to keep red. Recorded as pH0; then set the pH gradient to pH0-0.6, pH0-0.3, pH0, pH0+0.3, pH0+0.6, pH0+1, repeat the previous procedure until the lowest pH at room temperature for 2 h remains red.
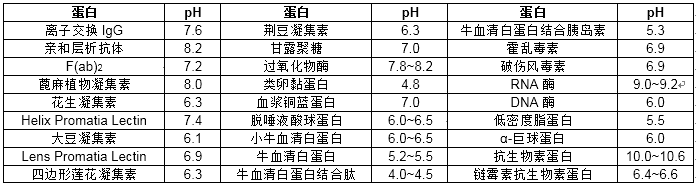
Generally, the optimal pH of the labeled antibody IgG is 9.0, the monoclonal antibody is 8.2, the SPA (proteinA) is 5.9-6.2, and the other commonly used protein labeled pH is shown in the following table:
B protein concentration optimization
After optimization of the pH value, continue to optimize the labeling of the lowest protein / antibody concentration, also using the gradient method, mixed with different concentrations of protein / antibody solution and colloidal gold (already in the best pH environment) solution, room temperature for 15 min, add 10% The sodium chloride was allowed to stand at room temperature for 2 h, and the OD520~580 was measured. The absorbance value was plotted against the protein/antibody concentration, and the protein/antibody concentration at the intersection of the linear trend line and the horizontal axis was the minimum protein/antibody concentration.
Once the optimum pH and minimum protein/antibody concentration have been determined, gold labeling can begin. In the pH-adjusted colloidal gold solution, the protein/antibody was added dropwise with rapid stirring (concentration was 1.2 times the previously determined minimum value), and the addition was completed within 5 min, and then 10% BSA was added to a final concentration of BSA of 1%. Stir for 10 min. Low temperature ultracentrifugation to remove unlabeled protein/antibody and under-labeled colloidal gold. For details, please refer to: first centrifuge at 1500 rpm for 1 h to remove the precipitate, then centrifuge at 15000 rpm for 1 h to remove the supernatant. Precipitate with TBS containing 1% BSA. Dissolve and repeat high-speed centrifugation three times to obtain a gold-labeled protein/antibody solution that can be used for the preparation of test strips.
C parameter optimization (partial condition optimization example)
1 Combination of sample pad and gold standard pad The pH of the sample pad treatment solution is set to three gradients (7.2, 8.0, 9.0), and the gold standard protein/antibody solution is set to three dilution factors (1:1, 1:2, 1:1: 3) The gold standard pad was prepared, and the coloration after the positive and negative control (saline) was observed by cross-combination (the T-line and the C-line were all coated at a concentration of 1 mg/mL).

Here we mainly optimize the pH of the sample pad treatment solution and the concentration of the gold standard substance. In addition, we can optimize the composition of the sample pad and the gold standard pad. This requires designing various combinations for testing.
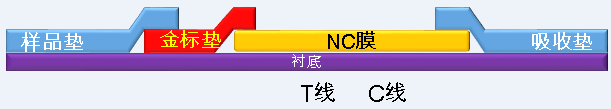
2 T line, C line coating concentration optimization
The antibody to be coated was diluted 1:1, 1:2, 1:3, coated with NC membrane, and then detected by positive and negative control (physiological saline) to observe the coloration, and the optimal dilution factor (concentration) was selected.
The antigen to be coated was diluted 1:10, 1:20, 1:40, 1:50, 1:100, and the antibody concentration was adjusted to the concentration of the previous step, coated with NC membrane, positive and negative control (normal saline) ) Detect and observe the color development and select the optimal dilution factor (concentration).
Of course, we can also use a combination test to optimize the concentration of the two coatings.
3 detection pad closure time optimization
The detection pad sealing time was set to four gradients of 1 h, 0.5 h, 1 h, and 2 h. The test strips were assembled with the parameters optimized before, and the positive and negative controls (saline) were added to detect the color development, and the optimal closure was selected. time. At the same time, we can also optimize the concentration of BSA, optimize the selection of coating materials, or optimize the combination of multiple parameters.
two. Application of immunocolloidal gold labeling technique Early immunogold gold labeling technique was mainly applied to the observation of cell surface antigen by optical microscopy. However, it was limited due to low sensitivity. After the establishment of IGSS, immunogold labeling technology was widely used. At present, the application of immunocolloidal gold labeling technology in histochemistry has become more and more popular, and the types of colloidal gold probes are also increasing. Because the high electron density of colloidal gold makes it clearly identifiable under electron microscopy, the technique is further used on electron microscopy. From the study of tissue cell localization, the gene transcription level, gene expression detection, chromosome gene location, and specificity are further advanced. The spatial distribution analysis of nucleic acid sequences in cells and the qualitative and quantitative analysis of nucleic acid replication processes.